Introduction
Angioimmunoblastic T-cell lymphoma (AITL) is the second most common subtype of peripheral T-cell lymphoma (PTCL). The therapeutic effect of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), the most common first-line chemotherapy regimen, is not satisfactory, and most patients experience relapse or disease progression. The 5-year progression-free survival (PFS) of AITL is 13%-23%, and 5-year overall survival (OS) is 33%-36%. The benzamide histone deacetylase inhibitor (HDACi) Chidamide and lenalidomide (a second-generation immunomodulatory compound) are both approved for the treatment of relapsed or refractory PTCL in China. About 70%-100% of the AITL patients have EBV infection, which may cause abnormal immune functions in hosts. EBV infects immunocytes such as B cells, T cells, and natural killer cells, and it impairs immune functions that can clear neoplastic cells from the body. Rituximab may eradicate EBV-B cells and activated B cells and may prevent the progression to AITL. When combined with rituximab, lenalidomide augmented antibody-dependent cell-mediated cytotoxicity by enhancing NK cells, which overcomes rituximab resistance in lymphoma patients. In this prospective phase II study, the efficacy and safety of a new regimen consisting of rituximab, lenalidomide and chidamide has been evaluated in relapsed/refractory AITL patients. We have reported the preliminary results (2022 ASH, poster 1600), and now we update the data of this study.
Methods
Patients with relapsed/refractory AITL after at least first-line chemotherapy were treated with up to 6 cycles of a rituximab and lenalidomide plus chidamide (RLC) regimen every 3 weeks. The initial dose of each drug has been reported. The primary endpoint of the study was PFS. The secondary endpoints included overall response rate (ORR), OS, and adverse events (AEs).
Results
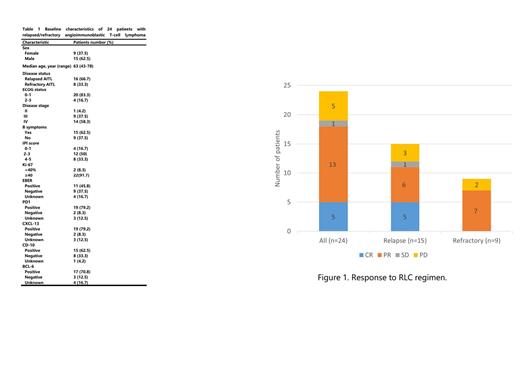
Between Aug 2019 and May 2023, 24 patients with relapsed/refractory AITL were enrolled. The characteristics of these patients are listed in table 1. On enrollment, 16 (66.7%) patients had received 1st-line chemotherapy, 7 (29.1%) patients had received 2nd-line chemotherapy, and 1 (4.2%) patient had received 3rd-line chemotherapy. The median age was 63 years (range 43-78) and 9 (37.5%) patients were female. Fifteen (62.5%) patients had B symptoms, including weight loss in 3 (12.5%) cases, night sweat in 1 (4.2%) case and fever in 11 (45.8%) cases. Sixteen (66.7%) patients had elevated serum lactate dehydrogenase (LDH), and 21 (87.5%) patients had IPI score≥2. All patients had completed RLC treatment for at least 1 cycle and underwent imaging assessment. The ORR was 75% (18/24) with a complete remission (CR) rate of 20.8% (5/24), and 13 (54.2%) patients achieved partial remission (PR). While 1 (4.2%) patient achieved stable disease (SD) and 5 (20.8%) patients achieved progressive disease (PD). Two patients received autologous hematopoietic stem cell transplantation as consolidation treatment after achieving CR from RLC chemotherapy. With a median follow-up of 9.7 months, 9 patients had died. The median PFS was 10.8 months (95% confidence interval <CI>, 9.0 to 12.6 months), but the median OS was not reached.
No patient presented with Grade 5 AE. The most frequent all-grade hematological toxicity included leucopenia (35.7%, 5/14), followed by anemia (21.4%, 3/14) and thrombocytopenia (41.7%, 10/24). Other common toxicities included infusion reaction (16.7%, 4/24), skin rash (20.8%, 5/24), pulmonary pneumonia (41.7%, 10/24), and nausea (12.5%, 3/24). Dose reduction was performed in 8 patients. The exploratory analysis of possible prognostic factors by mass spectrum flow are undergoing.
Conclusions
The updated results of this prospective study confirmed the good response rate and manageable toxicity of rituximab and lenalidomide plus chidamide (RLC) regimen. This combination may be a promising regimen in patients with relapsed/refractory AITL.
Disclosures
No relevant conflicts of interest to declare.